Abstract
Introduction Treatment of chronic phase (CP) chronic myeloid leukemia (CML) with tyrosine kinase inhibitors (TKIs) proved to be almost equally effective in young and elderly patients. Three TKIs, imatinib (IM), dasatinib (DAS) and nilotinib (NIL), are approved for frontline therapy in Italy. Choice of frontline TKI is based on a combined evaluation of patient's characteristics and expectations, with age usually playing a prominent role. However, to date, few data are available on patterns of TKI selection in very elderly patients.
Aim To analyse the use of frontline TKI therapy in a large and unselected cohort of very elderly CP-CML patients
Methods We retrospectively evaluated 332 patients aged ≥75 year diagnosed from 1/2012 to 12/2019 at 36 Hematology Centres participating at the "Campus CML" project.
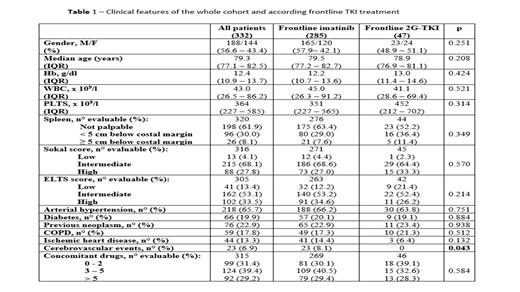
Results Clinical features at diagnosis for the whole cohort and according to frontline TKI are reported in Table 1. As to frontline TKI, 285 patients (85.8%) received IM and 47 (14.2%) a 2G-TKI (DAS n=28, 59.5%; NIL n=19, 40.5%). Of the 285 IM-treated patients, 192 (67.3%) started with standard dose (400 mg/day) and 93 (32.7%) with a reduced dose (300 mg/day n=64, 22.5%; <300 mg/day n=29, 10.2%). Among the 47 patients starting a 2G-TKIs, 35 (74.4%) received standard dose and 12 (25.6%) a reduced dose (NIL <600 mg/day n=3; DAS 80 mg/day n=4 and 50 mg/day n=5). There were no differences between patients treated with imatinib or 2G-TKI (Table 1); only a previous cerebrovascular event was reported in a significantly higher rate of IM-treated patients. It is however evident that the distinct toxicity profiles of NIL and DAS had an impact on TKI choice as, for example, no patient with diabetes or ischemic heart disease received NIL.
Following widespread introduction of generic IM in Italy in early 2018, patients were divided in 2 groups: among 238 patients diagnosed from 2012 to 2017, 198 (83.1%) received IM and 40 (16.9%) a 2G-TKI, while patients diagnosed in 2018-2019 were treated with IM in 87/94 (92.5%) cases and with a 2G-TKI in 7 (7.5%) cases only (p=0.028).
Conclusions IM remains the frontline drug of choice in very elderly CML patients, and this trend seems to increase after the introduction of the generic formulation. However, 2G-TKI are used in a small but sizeable group of patients, without a clear correlation with baseline CML features, thus probably reflecting a physician's evaluation of patient's fitness and/or expectation. Efficacy and safety of initial reduced TKIs doses in the setting of very elderly patients warrant further analyses.
Latagliata: Novartis: Honoraria; BMS Cellgene: Honoraria; Pfizer: Honoraria. Bonifacio: Novartis: Honoraria; Pfizer: Honoraria; Amgen: Honoraria; Bristol Myers Squibb: Honoraria. Elena: CELGENE: Other: funding for meeting participation; PFIZER: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Membership on an entity's Board of Directors or advisory committees; GILEAD: Membership on an entity's Board of Directors or advisory committees. Iurlo: Novartis: Speakers Bureau; Incyte: Speakers Bureau; Pfizer: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau. Sportoletti: AstraZeneca: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Stagno: Pfizer: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; InCyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Other: Support for attending meetings and/or travel, Research Funding. Abruzzese: Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria. Breccia: Bristol Myers Squibb/Celgene: Honoraria; Incyte: Honoraria; Abbvie: Honoraria; Pfizer: Honoraria; Novartis: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal